Abstract
Introduction
Emicizumab, a bispecific humanized monoclonal antibody administered subcutaneously, bridges FIXa and FX to restore the function of missing FVIIIa, and is being developed to prevent bleeds in patients with hemophilia A (PwHA) with and without inhibitors. An interim analysis of the HAVEN 2 study (n=20) in patients aged 2-12 years (data cutoff 28 Oct, 2016) showed that subcutaneous, once-weekly emicizumab prophylaxis successfully prevented or reduced bleeds, provided clinically meaningful reductions in annualized bleed rate (ABR) versus prior bypassing agent (BPA) treatment, and was well tolerated (Young et al. RPTH 2017;1 (S2):Abstract OC 24.1). Here we present an updated, much larger (40 additional patients, 60 total) analysis of efficacy, safety and pharmacokinetics (PK) of once-weekly subcutaneous (SC) emicizumab prophylaxis in pediatric PwHA with inhibitors.
Methods
The study (NCT02795767) enrolled PwHA with inhibitors aged 2-12 years (or 12-17 years if <40 kg), and currently enrolling those <2 years of age, previously treated with BPAs to receive emicizumab prophylaxis for ≥52 weeks. Efficacy analyses included ABR and bleed reduction vs ABR on prior BPA treatment from prospective non-interventional study (NIS; NCT02476942). Health-related quality of life (HRQoL), aspects of caregiver burden and safety parameters were also assessed.
Results
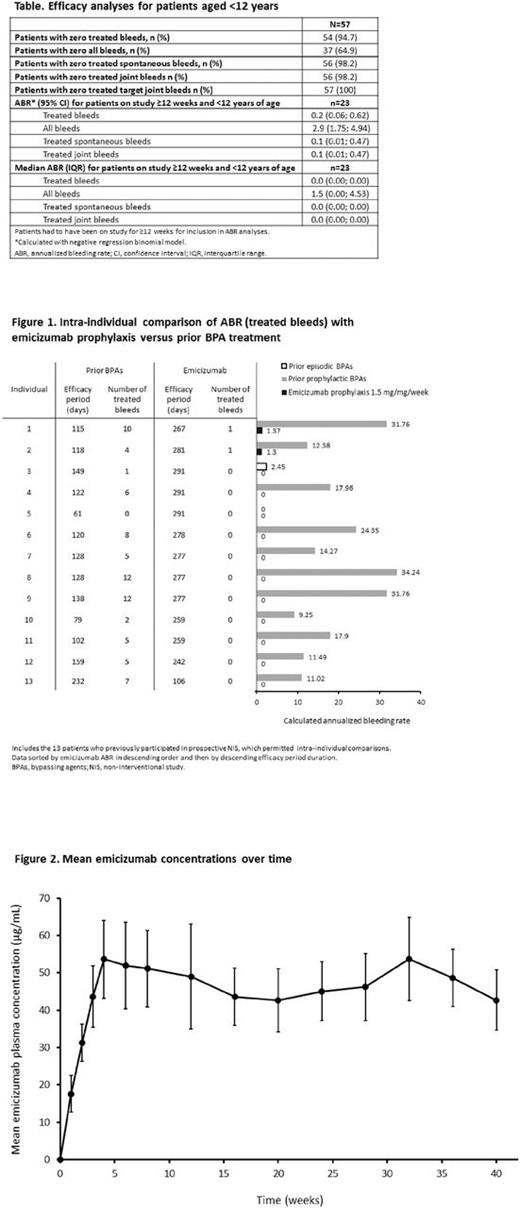
The updated analysis (8 May, 2017 cutoff) included approximately 6 additional months of data vs the first interim analysis; 60 PwHA with inhibitors aged 1-15 (median 7) years; 57 aged <12 years including 2 aged <2 years were included in the efficacy analyses. Three patients aged ≥12 years and <40kg were enrolled. The median observation time was 9 (range 1.6-41.6) weeks; 20 patients had been observed ≥24 weeks, and 2 patients aged <2 years for approximately 5 and 2 weeks. Efficacy data for patients aged <12 years are shown in the Table. Overall, 54/57 (94.7%) patients had zero treated bleeds. Only 3 treated bleeds were reported, with 1 occurring in a joint, 1 occurring in a muscle, and 1 hip bleed that was classified as "other"; all were safely treated with rFVIIa. Only 1 of these 3 treated bleeds was a spontaneous bleed. In total, 37/57 patients (64.9%) reported no bleeds. A total of 65 bleeds were reported in 20 patients, with 8 occurring in a joint, 2 occurring in a muscle, and 55 being classified as "other"; of the 55 "other'' bleeds, 26 (40.0%) were spontaneous, 36 (55.4%) traumatic and 3 (4.6%) due to procedure/surgery.
Twenty-three patients <12 years of age were followed for ≥12 weeks and therefore included in the ABR population calculation. The ABR was 0.2 (95% CI 0.06; 0.62) for treated bleeds (Table). Eighteen patients <12 years old had previously participated in the NIS. Of these, 13 patients had been on HAVEN 2 for ≥12 weeks and were therefore included in the intra-individual comparison; a substantial reduction in ABR of 99% with emicizumab prophylaxis vs prior BPA treatment was observed in these patients. Individual patient data are shown in Fig 1.
Considerable improvements in HRQoL, and aspects of caregiver burden were observed.
Emicizumab was well tolerated; the most common AEs were viral upper respiratory tract infection and injection site reactions (16.7% of patients each). Six patients experienced 7 serious AEs (2 muscle hemorrhage, 1 eye pain, 1 catheter site infection, 1 device-related infection, 1 mouth hemorrhage, 1 appendicitis), with none deemed related to emicizumab; no thromboembolic or thrombotic microangiopathy events were reported. No patients tested positive for anti-drug antibodies. Mean steady state trough emicizumab concentrations of approximately 50 µg/mL were maintained with longer follow-up (Fig 2). PK profiles were consistent across age groups and body weight.
Conclusion
HAVEN 2 is the largest study in pediatric PwHA with inhibitors to date, and demonstrates that emicizumab prophylaxis prevented or substantially reduced bleeds and was well tolerated in this patient population. PK remained consistent with that seen in adolescent/adult PwHA. Weekly subcutaneous emicizumab has the potential to reduce overall treatment and disease burden and may provide a new standard of care for hemophilia management by providing an effective, safe and convenient option for pediatric PwHA with inhibitors.
Young: CSL Behring: Honoraria; Novo Nordisk: Consultancy. Sidonio: Bioverativ: Research Funding; Novo Nordisk: Consultancy; Shire: Consultancy, Research Funding; Grifols: Research Funding; CSL Behring: Consultancy; Bioverativ: Consultancy; Bayer: Consultancy. Liesner: NovoNordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Baxalta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; SOBI: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Octapharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bio Products Laboratory: Consultancy, Membership on an entity's Board of Directors or advisory committees; SOBI/Bioverativ: Research Funding, Speakers Bureau; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Oldenburg: Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Chugai: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Octapharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Grifols: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Baxter: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Biotest: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy, Honoraria, Investigator Clinical Studies and Research Funding, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Biogen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Baxalta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Investigator Clinical Studies and Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Chang: Genentech: Employment, Equity Ownership. Uguen: F. Hoffmann-La Roche Ltd: Employment. Dhalluin: F. Hoffmann-La Roche Ltd: Employment. Schmitt: F. Hoffmann-La Roche Ltd: Employment. Levy: Genentech, Inc.: Employment. Shima: Pfizer: Honoraria, Research Funding; Baxalta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Chugai: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL: Honoraria, Research Funding; Biogen: Consultancy, Honoraria; Kaketsuken: Honoraria; Novo: Honoraria, Research Funding; Bayer: Honoraria, Research Funding. Mahlangu: Catalyst Biosciences: Consultancy, Research Funding; Baxalta: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Biogen: Research Funding, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Biotest: Speakers Bureau; Bayer: Research Funding, Speakers Bureau; Alnylam: Consultancy, Research Funding, Speakers Bureau; Baxalta: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alnylam: Consultancy, Research Funding, Speakers Bureau; CSL Behring: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bayer: Research Funding, Speakers Bureau; NovoNordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Biotest: Speakers Bureau; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Biogen: Research Funding, Speakers Bureau; Shire: Consultancy, Research Funding, Speakers Bureau; Sobi: Research Funding, Speakers Bureau; Catalyst Biosciences: Consultancy, Research Funding; CSL Behring: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; NovoNordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Shire: Consultancy, Research Funding, Speakers Bureau; Sobi: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal